Cyber Security
In the increasingly interconnected and digitized healthcare industry, users and manufacturers face significant challenges. The expanding attack surface and growing system complexity require a structured approach to cybersecurity. A systematic approach is crucial to meet the rising demands for the security of medical products.
To meet these demands, we at softgate GmbH ensure our cybersecurity measures adhere to current standards:
Our standards
Security Process Description
Using the ANSI/AAMI SW96:2023 standard, we systematically identify and address potential threats during the design phase. This involves a structured approach to risk assessment and management to ensure comprehensive security coverage throughout the product lifecycle.. We emphasize adherence to proven security principles throughout the development process, including "Least Privilege," "Defense in Depth," and "Security by Design." Our stringent coding practices minimize vulnerabilities and ensure that our software is robust and resilient against attacks. We conduct continuous monitoring and regular security scans to quickly identify and address potential threats, keeping systems secure and compliant. We utilize techniques such as static and dynamic analysis, as well as advanced security testing, to evaluate and enhance the implementation of security requirements in our products.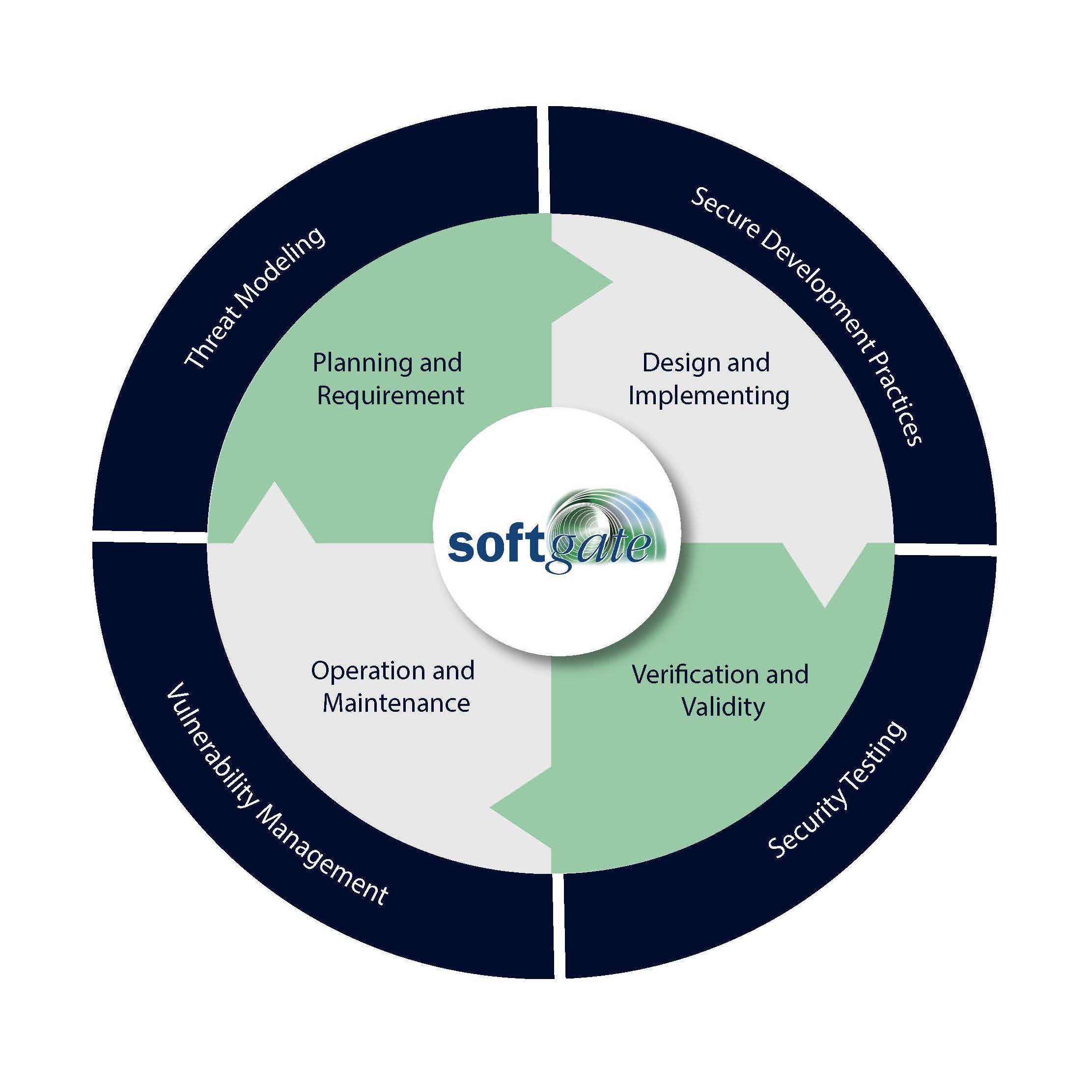
Threat Modeling
Secure Development Practices
Vulnerability Management:
Security Testing
Our Expertise
With many years of experience in cybersecurity for medical products, our team collaborates successfully with leading industry partners and is involved in all process steps—from initial planning and development to risk management, implementation, and continuous monitoring. Below are some examples of our successful projects.
Support during complete SDLC
SBOM based vulnerability analysis
Contact us:
Are you planning a new project? Whether you’re building a new application or enhancing an existing one, we provide the expertise to efficiently implement robust, secure, and high-performance software solutions for medical technology.